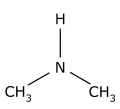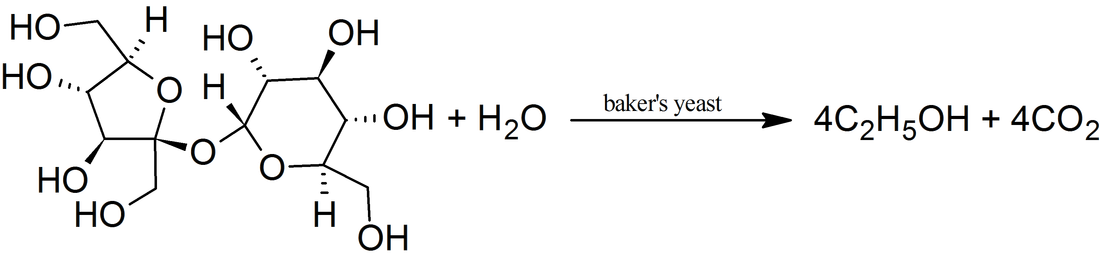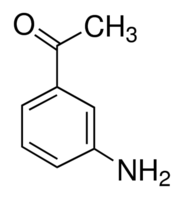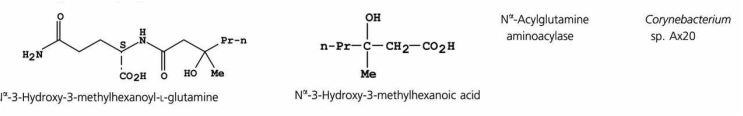
Toluene can be found in urine, blood, feces, saliva and breath. Background levels of toluene in breath measured in toluene-exposed workers are about 1,000-fold higher than in non-exposed individuals.
Toluene metabolites (Hippuric acid and cresols) can be also used as indicators of exposure.