3-hydroxy-3-methylhexanoic acid (HMHA) is a branched fatty acid detected relatively in abundance in sweat. It has two enantiomers - right handed (R) and left-handed (L).
(+)-(S)-3-hydroxy-3-methylhexanoic acid - Armpit odor like and cumin spice like odor; also described by Hasegawa et. al. as having a strong spicy odor
(-)-(R)-3-hydroxy-3-methylhexanoic acid - Cumin spice like odor, weaker than racemic body; also described by Hasegawa et. al. as having a weak animalic odor.
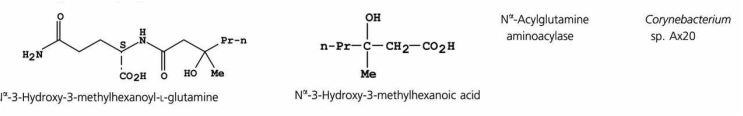
Since the early 1990s, structurally unusual medium-chain (C6–C10) Volatile Fatty Acids (VFAs), in particular the trans (E) isomer of 3-methyl-2-hexenoic acid (3M2H), have been shown to contribute to underarm odor. Subsequently, it was found that 3M2H, and the structurally related 3-hydroxy-3-methylhexanoic acid, are released by the action of a corynebacterial enzyme, Nα-acylglutamine aminoacylase, highly specific for the glutamine residue. A wide range of medium-chain VFAs are present in apocrine sweat released on skin by corynebacteria, with 3-hydroxy-3-methylhexanoic acid and 3M2H being the dominant species in terms of relative abundance.
Women have the potential to liberate significantly more (R)/(S)-3-methyl-3-sulfanylhexan-1-ol - (R)/(S)-MSH. - which has a tropical fruit- and onion-like odor than (R)/(S)-3-hydroxy-3-methylhexanoic acid - (R)/(S)-HMHA - (possibly transformed into (E)/(Z)-3-methyl-2-hexenoic acid) that has a cheesy, rancid odor.
A fatty acid with one less Carbon - 2-Hydroxy-3-methylpentanoic acid generated by L-isoleucine metabolism is elevated in the urine and blood of patients with maple syrup urine disease, leading to a distinctive sugary maple syrup odor.
