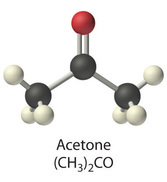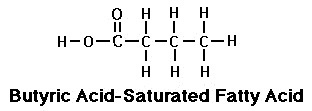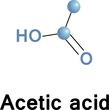
One of short chain fatty acids (SCFAs, also called volatile fatty acids) containing fewer than 6 carbons: Formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), and valeric acid (C5). The gut microbial metabolites SCFAs profoundly regulate T cell differentiation in the body, boosting immune system.
if raised, suggests bacterial fermentation due to excess carbohydrate reaching the colon; levels are not significantly altered in patients with malignancy or diabetes mellitus, but severe liver disease and severe acidosis were both associated with increased acetate concentrations. Elevated blood acetate is also an indicator of fast ethanol elimination in chronic alcoholics.